Nickel, a versatile transition metal with atomic number 28 in the periodic table, is renowned for its unique properties that make it an indispensable element in various industrial, technological, and scientific applications. One of the most intriguing aspects of nickel is its electrical conductivity. In this article, we delve into the world of nickel’s electrical properties to explore the fundamental question: Is nickel conductive?
Understanding the factors that influence nickel’s electrical conductivity is crucial in appreciating its significance in various applications. Temperature plays a critical role in determining the efficiency of electrical conduction in nickel. With the rising temperature, the thermal vibrations of atoms and electron-lattice interactions increase, leading to greater scattering of electrons and subsequently a rise in resistivity.
Additionally, the presence of impurities or doping can alter the concentration of charge carriers, influencing the material’s conductivity. This article explores how different temperatures and doping affect nickel’s conductivity, shedding light on the practical implications of these factors in real-world applications.
Moreover, we explore how nickel compares to other conductive materials, such as copper and silver, renowned for their high electrical conductivity. A comprehensive examination of nickel’s electrical properties against these metals offers insights into its positioning as a conductor and the rationale behind its selection in specific scenarios.
Structure And Bonding Of Nickel
Crystal Structure
Nickel has a face-centered cubic (FCC) crystal structure, which is a common arrangement for many metallic elements. In this structure, each nickel atom is positioned at the corners of a cube and at the center of each face of the cube. The arrangement of atoms in an FCC lattice provides several advantages, including high ductility, thermal stability, and electrical conductivity.

Bonding in Nickel
Nickel’s bonding is primarily metallic in nature. Metallic bonds are formed by the sharing of electrons between neighboring atoms within the crystal lattice. In the case of nickel, the outer electron configuration is [Ar] 3d^8 4s^2, and it has a strong affinity to lose its two outermost electrons to achieve a stable electron configuration.
As nickel atoms come together in a crystal lattice, their outermost electrons delocalize and form a “sea” of electrons that are free to move throughout the entire lattice. These delocalized electrons are responsible for the metal’s excellent electrical conductivity. Furthermore, the strong bonding between the positively charged nickel ions and the negatively charged delocalized electrons contributes to nickel’s exceptional mechanical properties, such as strength and malleability.
Alloys and Applications
One of the most significant uses of nickel is in the production of alloys. Nickel alloys are created by combining nickel with other elements, such as chromium, iron, or copper. These alloys exhibit enhanced properties, including improved corrosion resistance, higher temperature strength, and increased durability. The addition of nickel to stainless steel, for example, enhances its resistance to rust and corrosion, making it ideal for applications in harsh environments like marine and aerospace industries [2].
Nickel-based superalloys are another critical class of materials where nickel’s properties shine. These alloys can withstand extreme temperatures and mechanical stress, making them suitable for use in gas turbines for power generation and aircraft engines. Their ability to retain strength and resist deformation at high temperatures is vital in ensuring the efficient and safe operation of jet engines and power plants.
Catalysis and Chemical Compounds
Nickel is also a vital component of many catalysts used in various chemical reactions. Nickel catalysts are employed in processes such as hydrogenation, where hydrogen gas is added to unsaturated compounds to produce valuable chemicals. The unique electronic structure of nickel, coupled with its ability to form multiple oxidation states, makes it an efficient catalyst for a wide range of reactions in the chemical industry.
In addition to its role in catalysis, nickel forms various chemical compounds, including nickel oxide and nickel chloride, which have their specific uses in industry and research.

Properties Of Nickel
1. High Melting Point and Density
Nickel has a relatively high melting point of around 1453°C (2647°F), making it suitable for applications that involve high temperatures. Its density is approximately 8.91 grams per cubic centimeter, making it a relatively heavy metal with good weight-to-strength ratio.
2. Excellent Corrosion Resistance
One of the most notable properties of nickel is its exceptional resistance to corrosion. When exposed to air or moisture, nickel forms a thin oxide layer on its surface, which protects it from further oxidation. This property makes nickel highly desirable for use in various environments where corrosion resistance is essential, such as marine and chemical industries.
3. Ductility and Malleability
Nickel is a highly ductile and malleable metal, meaning it can be easily drawn into wires or hammered into thin sheets without breaking. These properties are crucial in manufacturing processes where the metal needs to be shaped into specific forms for various applications.
4. Ferromagnetic Property
Nickel is ferromagnetic at room temperature, meaning it exhibits a permanent magnetic moment and can be easily magnetized. This property is valuable in the production of magnets for electric motors, generators, and other magnetic devices [3].
5. Good Electrical Conductivity
Nickel is an excellent electrical conductor due to the presence of delocalized electrons in its metallic bonding. It is commonly used in electrical contacts, connectors, and wiring in various electronic and electrical devices.
6. High-Temperature Strength
Nickel retains its mechanical strength and integrity at elevated temperatures, which makes it ideal for applications in high-temperature environments. Nickel-based superalloys are extensively used in gas turbines, jet engines, and other aerospace applications due to their ability to withstand extreme temperatures and mechanical stress.

7. Catalytic Properties
Nickel is widely used as a catalyst in various chemical reactions, particularly in hydrogenation processes, where hydrogen gas is added to unsaturated compounds. Its catalytic properties arise from its ability to change oxidation states and its unique electronic structure.
8. Alloy Formation
Nickel readily forms alloys with many other metals, including iron, copper, and chromium. These alloys often exhibit improved properties, such as increased strength, corrosion resistance, and high-temperature performance. Stainless steel, which contains nickel as one of its main components, is a prime example of a widely used nickel alloy.
9. Radioactive Isotopes
Nickel has several radioactive isotopes, with nickel-63 being the most stable one. These isotopes find applications in various fields, including radioactive dating, tracer studies, and medical imaging.
Uses Of Nickel
Stainless Steel Production
One of the most significant uses of nickel is in the production of stainless steel. Nickel is a key component of stainless steel alloys, typically comprising around 8-25% of the alloy composition. The addition of nickel enhances the corrosion resistance, durability, and strength of stainless steel, making it ideal for applications in kitchenware, cutlery, construction, and architecture.
Aerospace and Gas Turbines
Nickel-based super-alloys play a crucial role in the aerospace and gas turbine industries. These alloys can withstand high temperatures and mechanical stress, making them suitable for use in jet engines, gas turbines, and other aerospace components.
Electronics and Electrical Equipment
Nickel’s excellent electrical conductivity makes it valuable in electronics and electrical equipment. It is used in electrical contacts, connectors, and wiring for various devices, including smartphones, computers, appliances, and power generation equipment.

Batteries
Nickel is used in the manufacturing of rechargeable batteries, particularly nickel-cadmium (Ni-Cd) and nickel-metal hydride (Ni-MH) batteries. These batteries are commonly found in portable electronic devices, power tools, and hybrid vehicles.
Chemical Industry
Nickel is an essential component in catalysts used in various chemical reactions. Nickel catalysts are employed in hydrogenation processes, where hydrogen gas is added to unsaturated compounds to produce valuable chemicals. These catalysts are used in the production of margarine, vegetable oils, and pharmaceuticals.
Coins
Nickel is used in coin production due to its durability, resistance to corrosion, and ability to maintain a polished appearance. Many countries use nickel in the production of coins, especially for lower denominations.
Medical Devices
Nickel is used in various medical devices and equipment, including implants, orthodontic braces, and surgical instruments. Its biocompatibility and resistance to corrosion make it suitable for these applications.
Marine and Naval Applications
Due to its exceptional corrosion resistance, nickel is used in marine and naval applications, such as shipbuilding, offshore platforms, and seawater desalination plants. Nickel alloys provide excellent protection against saltwater corrosion and degradation [4].
Plating and Coatings
Nickel plating is widely used to provide a protective and decorative coating to various objects, such as automotive parts, household items, and jewelry. The plated nickel layer enhances the object’s appearance and prevents corrosion.
Alloys and Special Steels
Nickel is alloyed with other metals, such as copper, iron, and chromium, to create special steels with specific properties. These alloys find applications in the automotive industry, construction, and machinery manufacturing.

What Is Electrical Conductivity?
Electrical conductivity, also known as electrical conductance, is a fundamental property of materials that describes their ability to conduct electric current. It is a measure of how easily electric charges can move through a material in response to an applied electric field. In other words, it quantifies how well a material can transmit electricity.
Electric current is the flow of electric charge, typically carried by electrons in a conducting material. When an electric potential (voltage) is applied across a conductor (a material with high electrical conductivity), electrons are compelled to move in the direction of the applied electric field. This movement of electrons constitutes an electric current.
The electrical conductivity of a material depends on its atomic or molecular structure and the availability of charge carriers. Charge carriers are particles, such as electrons or ions, that are responsible for carrying electric charge through the material. The higher the concentration of charge carriers and their mobility within the material, the better the electrical conductivity.
Materials can be broadly classified into three categories based on their electrical conductivity:
- Conductors: Materials with high electrical conductivity. They have a large number of mobile charge carriers, typically electrons, and allow electric current to flow easily. Common examples of conductors include metals like copper, aluminum, silver, and gold;
- Insulators: Materials with very low electrical conductivity. They have very few mobile charge carriers and impede the flow of electric current. Examples of insulators include rubber, glass, plastic, and wood;
- Semiconductors: Materials with intermediate electrical conductivity. Semiconductors have a moderate number of charge carriers, and their conductivity can be greatly influenced by factors such as temperature and impurities. Silicon and germanium are well-known semiconductor materials and are extensively used in electronic devices;
Electrical conductivity is an essential property in various applications. High-conductivity materials are used in electrical wiring, power transmission lines, electronic circuits, and electrical appliances. On the other hand, insulators are used to protect against electric shocks and to insulate electrical components from each other. Semiconductors are at the core of modern electronics and form the basis of transistors, diodes, and integrated circuits.
The electrical conductivity of a material is typically measured in units of siemens per meter (S/m) or its inverse, ohm-meter (Ω·m). Materials with higher electrical conductivity have lower resistivity and allow electric current to flow more easily, while materials with lower electrical conductivity have higher resistivity and impede the flow of electric current [5].
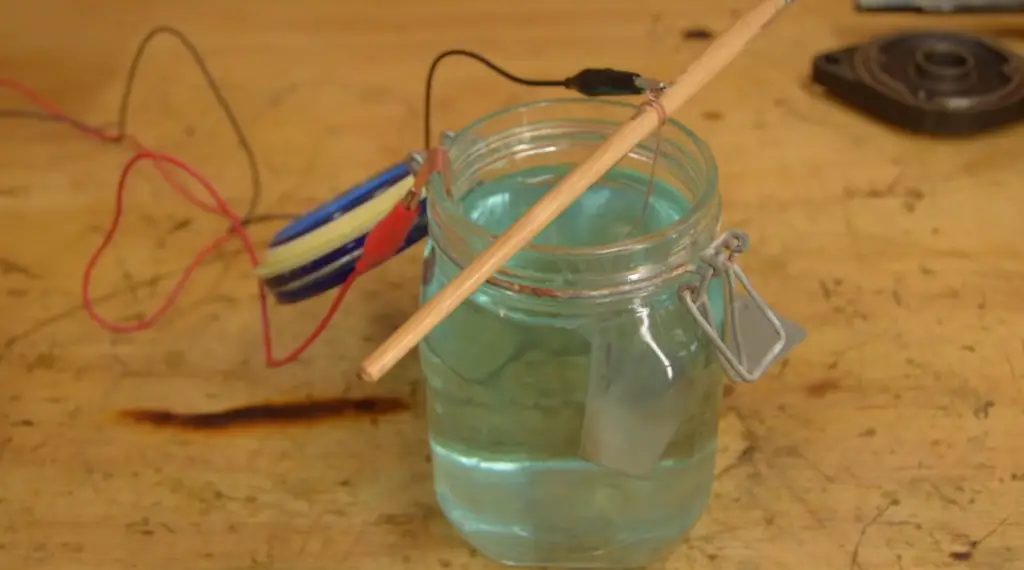
Does Nickel Conduct Electricity?
Yes, nickel is a good conductor of electricity. As a metal, nickel possesses high electrical conductivity due to its unique atomic structure and metallic bonding. In metals like nickel, the outermost electrons of the atoms become delocalized, meaning they are not bound to any specific atom but are free to move throughout the entire material.
When an electric potential (voltage) is applied across a piece of nickel, these delocalized electrons respond to the electric field and move in the direction of the applied voltage. This movement of electrons constitutes an electric current, and it is the reason why nickel, like other conductive metals, can transmit electricity efficiently.
Nickel’s electrical conductivity, along with its other valuable properties like corrosion resistance and strength, makes it a valuable material in various electrical and electronic applications. It is used in electrical contacts, connectors, and wiring in electronic devices, power generation equipment, and many other electrical systems. Additionally, nickel’s conductivity also plays a crucial role in its applications as an alloying element in stainless steel and other metal alloys.
Electrical Conductivity And Resistivity Of Nickel
Nickel is a good conductor of electricity, and its electrical conductivity is relatively high compared to many other materials. The electrical conductivity of nickel is approximately 14.4 × 10^6 siemens per meter (S/m) at room temperature.
Resistivity is the inverse of electrical conductivity and is a measure of how strongly a material resists the flow of electric current. It is denoted by the symbol “ρ” (rho) and is measured in ohm-meters (Ω·m).
Resistivity can be calculated using the formula:
Resistivity (ρ) = 1 / Electrical Conductivity
For nickel, the resistivity can be calculated as:
ρ = 1 / 14.4 × 10^6 S/m ≈ 6.94 × 10^-8 Ω·m
As you can see, the resistivity of nickel is relatively low, which indicates that it is an excellent conductor of electricity. This property is one of the reasons why nickel is widely used in electrical contacts, wiring, and various electronic components where efficient electrical conductivity is crucial [6].
Keep in mind that the electrical conductivity and resistivity of materials can vary with temperature and other factors. The values provided above are approximate and typically represent room temperature conditions. In some specific applications, the electrical properties of nickel might be adjusted by alloying it with other elements to meet specific requirements.

Factors That Affect The Electrical Conductivity Of Nickel:
1. Atomic Structure
Nickel’s electrical conductivity is greatly influenced by its atomic structure. In metals, including nickel, the outermost electrons of the atoms become delocalized, creating a “sea” of free electrons. These delocalized electrons are responsible for conducting electricity through the material. The more electrons available for conduction and the easier they can move, the higher the electrical conductivity. Nickel’s atomic structure, with its eight valence electrons (in the 3d^8 4s^2 configuration), contributes to its excellent electrical conductivity.
2. Temperature
The temperature has a significant impact on the electrical conductivity of nickel. As the temperature increases, the thermal vibrations of the atoms and the collisions between electrons and lattice defects increase, which can impede the movement of electrons and increase the material’s resistivity.
Therefore, the electrical conductivity of nickel generally decreases with increasing temperature. For precision applications, it is essential to consider the temperature dependence of nickel’s electrical properties.
3. Doping or the Presence of Impurities
The addition of small amounts of other elements to nickel, known as doping, can alter its electrical conductivity. Doping can increase or decrease the number of charge carriers (electrons) or influence their mobility within the material.
For instance, intentional doping with certain elements can enhance electrical conductivity, making nickel more suitable for specific electrical and electronic applications. On the other hand, the presence of impurities can introduce scattering centers for electrons, reducing the material’s electrical conductivity.
4. Pressure
Pressure can also affect the electrical conductivity of nickel. Applying pressure can alter the atomic spacing and electronic structure, influencing the movement of electrons. In some cases, increased pressure may lead to an increase in electrical conductivity, while in others, it may have the opposite effect. However, the impact of pressure on electrical conductivity is generally less significant than the effect of temperature or doping.
5. Strain
Mechanical strain, caused by deformation or stress on the material, can modify the arrangement of atoms and the electron-lattice interactions in nickel. Strain can either increase or decrease the electrical conductivity, depending on the direction and magnitude of the applied stress. Understanding the response of nickel to strain is crucial for designing strain-sensitive devices and optimizing its performance in various applications.
Does Nickel Conduct Heat?
Yes, nickel is a good conductor of heat. As a metal, nickel possesses high thermal conductivity, meaning it can efficiently transfer heat from one point to another when there is a temperature difference. This property is due to the free movement of electrons in its atomic structure, which allows them to carry thermal energy throughout the material.
When heat is applied to one end of a piece of nickel, the atoms at that end gain energy and vibrate more vigorously. These energetic atoms then transfer some of their energy to neighboring atoms through collisions. This process continues, and the thermal energy propagates through the material as a wave of vibrating atoms.
Metals like nickel have a high thermal conductivity because of the presence of delocalized electrons. These electrons can move rapidly throughout the material, carrying thermal energy with them and facilitating efficient heat transfer. As a result, heat can easily flow through nickel, making it useful in various applications where heat needs to be conducted or dissipated.
The high thermal conductivity of nickel is utilized in several applications, including:
- Heat Exchangers: Nickel is used in the manufacture of heat exchangers, which transfer heat from one fluid to another without them mixing. These exchangers are commonly found in refrigeration systems, air conditioning units, and industrial processes;
- Electronics: Nickel is used as a component in electronic devices to dissipate heat generated during their operation. It is commonly found in heat sinks, which help cool electronic components such as CPUs and GPUs;
- Electrical Components: The high thermal conductivity of nickel is beneficial in electrical contacts and connectors, as it helps in distributing heat evenly and preventing hotspots;
- Alloys: Nickel’s thermal conductivity is valuable in various alloys used in high-temperature applications, such as gas turbine components and jet engines [7];
Is Nickel A Good Conductor Of Electricity:
Is Nickel Metal Conductive?
Yes, nickel metal is highly conductive. Being a metal, nickel exhibits metallic bonding, which results in the delocalization of its valence electrons. This characteristic allows nickel to conduct electricity effectively. As a result, nickel is commonly used in electrical applications where efficient electrical conductivity is required.
Is Nickel Non-Conductive?
Nickel is conductive. Non-conductive materials, also known as insulators, have very low electrical conductivity. Unlike insulators, metals like nickel have a large number of delocalized electrons that can move freely throughout the material, enabling the efficient transmission of electric current. This is in contrast to non-conductive materials that have tightly bound electrons, preventing the flow of electric charges.
Is Nickel an Insulator?
No, nickel is not an insulator; it is a conductor. As mentioned earlier, insulators have extremely low electrical conductivity and do not allow the easy flow of electric current. Nickel, being a metal with delocalized electrons, is a good conductor of electricity. It is an integral part of electrical and electronic devices, where its conductivity is harnessed for efficient transmission of electricity.
Is Molten Nickel A Good Conductor Of Electricity?
Yes, molten nickel is a good conductor of electricity. When a solid metal like nickel is melted, its atoms lose their fixed lattice structure, and the metallic bonds break, resulting in the delocalization of valence electrons. In the molten state, nickel retains its metallic nature, with free-moving electrons throughout the liquid.
The presence of delocalized electrons in molten nickel allows it to conduct electricity efficiently. When an electric potential (voltage) is applied across the molten nickel, these free electrons respond to the electric field and move in the direction of the applied voltage, just like they do in solid nickel. This movement of electrons constitutes an electric current, and molten nickel can easily transmit electricity.
The ability of molten nickel to conduct electricity is essential in various industrial processes and applications, especially in metallurgy and metal smelting. During the production of nickel or nickel-containing alloys, molten nickel can be used as a conductive medium to extract and refine metals from their ores.
It’s worth noting that working with molten metals, including molten nickel, requires special precautions due to the high temperatures involved and the potential for burns and other hazards. Proper safety measures must be followed in industrial processes involving molten nickel to ensure the well-being of workers and the integrity of the equipment.
FAQ:
1. Is nickel an insulator or a conductor?
Nickel is a conductor. As a metal, it possesses delocalized electrons in its atomic structure, allowing it to efficiently conduct electricity.
2. Why does nickel conduct electricity?
Nickel conducts electricity due to the presence of delocalized electrons. When an electric potential is applied, these free-moving electrons respond to the electric field and create an electric current through the material.
3. What is the electrical conductivity of nickel?
The electrical conductivity of nickel is approximately 14.4 × 10^6 siemens per meter (S/m) at room temperature.
4. Is nickel a good conductor of electricity?
Yes, nickel is a good conductor of electricity, thanks to its high electrical conductivity and delocalized electrons.
5. Is nickel a good conductor of heat and why?
Yes, nickel is a good conductor of heat. The presence of delocalized electrons allows thermal energy to be efficiently transferred through the material.
6. Do metals conduct electricity when molten?
Yes, metals, including nickel, continue to conduct electricity when they are in a molten state. The metallic bonding and the free movement of electrons persist even in the liquid phase.
7. Is nickel a weak conductor of electricity?
No, nickel is not a weak conductor of electricity. It has relatively high electrical conductivity, making it an effective conductor.
8. Is nickel a better conductor than copper?
No, copper is a better conductor of electricity than nickel. Copper has even higher electrical conductivity, which is why it is commonly used in electrical wiring and conductors.
9. Is nickel a better conductor than silver?
No, silver is a better conductor of electricity than nickel. Silver has the highest electrical conductivity among all metals, making it an excellent conductor.
10. Why is nickel used in wires?
Nickel is used in wires and electrical components due to its good electrical conductivity, resistance to corrosion, and ability to withstand high temperatures.
11. Is nickel more conductive than stainless steel?
Yes, nickel is more conductive than stainless steel. Stainless steel contains nickel as an alloying element, but its electrical conductivity is lower than that of pure nickel.
12. What is the best metal for conductivity?
Silver is the best metal for electrical conductivity, followed by copper.
13. Which is more conductive – nickel or copper?
Copper is more conductive than nickel.
14. Is nickel or gold more conductive?
Gold is less conductive than nickel, copper, and silver.
15. Is nickel more conductive than brass?
Yes, nickel is more conductive than brass.
16. What metal is a bad conductor of electricity?
Mercury is a metal that is a relatively poor conductor of electricity.
17. What is the weakness of nickel?
One weakness of nickel is that it can be prone to corrosion in certain environments.
18. Is nickel a cheap metal?
Nickel is not as cheap as some other metals like iron or aluminum, but its cost is relatively moderate.
19. Is nickel very magnetic?
Nickel is ferromagnetic, meaning it can become strongly magnetic when exposed to a magnetic field.
20. Is nickel the least effective conductor?
No, there are metals that are less effective conductors of electricity than nickel.
21. Is nickel reactive with air?
Nickel forms a thin oxide layer when exposed to air, which helps protect it from further oxidation. However, it can still react with certain substances under specific conditions.
22. Is zinc nickel-conductive?
Zinc is a fair conductor of electricity, but it is not as conductive as metals like copper or nickel.
23. Is nickel harder than steel?
Nickel is not inherently harder than steel, but it can be alloyed with other elements to create harder materials.
24. Is nickel stronger than aluminum?
Nickel is generally stronger than aluminum, especially at higher temperatures.
25. Is nickel wire safe?
Nickel wire is safe for many applications, but some individuals may have allergies or sensitivities to nickel, leading to skin reactions. For certain specialized uses, alternative materials may be preferred.
Useful Video: Which Metals Conduct Electricity The Best? | Metal Supermarkets
References
- https://tampasteel.com/best-metals-conduct-electricity/
- https://lambdageeks.com/does-nickel-conduct-electricity/
- https://blog.thepipingmart.com/metals/does-nickel-conduct-electricity/
- https://www.bluesea.com/resources/108/Electrical_Conductivity_of_Materials
- https://biotrux.com/is-nickel-a-conductor/
- https://www.circuitsgallery.com/is-nickel-conductive/
- https://www.sharrettsplating.com/blog/most-conductive-metals/










Leave a Reply