Saltwater, the mixture of water and dissolved salts, has long intrigued scientists and enthusiasts alike with its unique properties. One of the most fascinating characteristics of saltwater is its ability to conduct electricity.
Unlike pure water, which is known for its low electrical conductivity, saltwater’s conductivity opens the door to a myriad of applications in diverse fields, from powering electronic devices to facilitating industrial processes.
In this comprehensive article, we will delve into the science behind saltwater conductivity, exploring the factors that make it a conductor of electricity, the behavior of ions in the solution, and its comparison to other conductive and non-conductive mediums.
Furthermore, we will examine the applications of saltwater conductivity and how harnessing this property contributes to various cutting-edge technologies. Join us on this electrifying journey as we unravel the mysteries of saltwater conductivity and its vital role in shaping the world we live in today.
Structure And Properties Of Salt
Salt, scientifically known as sodium chloride (NaCl), is a fundamental compound that has played a crucial role in the development of human civilization. It is one of the most abundant and essential minerals found on Earth, and its unique structure and properties have made it a staple ingredient in various applications, from seasoning food to industrial processes.
The Structure of Salt
Salt’s structure is relatively simple but exhibits remarkable characteristics. It is composed of two essential elements, sodium (Na) and chlorine (Cl), held together by ionic bonds. Sodium is a metal, and chlorine is a halogen, making salt a representative example of an ionic compound. The ionic bond between these elements results from the transfer of an electron from sodium to chlorine, forming positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-) [1].
When a large number of sodium and chloride ions come together, they arrange themselves in a regular pattern, forming a crystal lattice structure. The ions are arranged in a repeating pattern, with each sodium ion surrounded by six chloride ions and vice versa. This ionic lattice structure gives the salt its characteristic cubic shape, often seen as tiny cubes or larger salt crystals.
Physical Properties of Salt
The unique structure of salt contributes to its diverse physical properties, some of which are as follows:
- Solubility: Salt is highly soluble in water and many other polar solvents. When added to water, the ionic bonds between sodium and chloride ions are broken, and the ions disperse throughout the solution. This property makes salt a crucial ingredient in various culinary and chemical processes;
- Melting and Boiling Points: Salt has a relatively high melting point at 801°C (1,474°F) and a boiling point of 1,413°C (2,575°F). This high heat resistance makes it valuable in metallurgy and other high-temperature applications;
- Conductivity: When dissolved in water, the salt solution becomes an excellent conductor of electricity due to the presence of free-moving ions. This property is essential for various industrial and laboratory processes;
- Hygroscopic Nature: Salt has a hygroscopic nature, meaning it can absorb moisture from the surrounding atmosphere. This property finds applications in preserving food and controlling humidity in certain environments [2];
Chemical Properties of Salt
While salt is primarily known for its role in enhancing the flavor of food, it also exhibits various chemical properties that contribute to its versatility:
- Neutral pH: A salt solution has a neutral pH of around 7. When dissolved in water, the sodium and chloride ions do not significantly alter the pH of the solution, making salt useful in maintaining proper pH levels in various applications;
- Reactivity: Though salt is relatively stable, it can undergo certain chemical reactions under specific conditions. For example, it can react with strong acids to form other compounds;
- Electrolysis: Salt is an essential component in the process of electrolysis, where an electric current is passed through a salt solution to produce chlorine gas, hydrogen gas, and sodium hydroxide;

Health Considerations
While salt is essential for maintaining proper bodily functions, excessive consumption can lead to health issues. High salt intake is associated with hypertension (high blood pressure), which increases the risk of heart disease and stroke. As a result, it is essential to consume salt in moderation and be aware of its presence in processed foods.
Uses Of Salt
Salt plays a vital role in various aspects of our daily lives, beyond its use as a seasoning in cooking. Some of its crucial applications include:
- Culinary Use: Salt has been used for centuries to enhance the flavor of food. It not only adds taste but also contributes to food preservation by creating an environment where bacteria and other microorganisms find it difficult to thrive;
- Water Treatment: In the water treatment industry, salt is used to soften hard water by removing calcium and magnesium ions. It is also employed in the treatment of pool water to maintain the proper chemical balance;
- Chemical Industry: The chemical industry relies heavily on salt for the production of numerous chemicals, including chlorine, sodium hydroxide, and various other sodium and chlorine compounds;
- De-icing: During winter, salt is commonly used to de-ice roads and sidewalks. When spread on icy surfaces, salt lowers the freezing point of water, causing the ice to melt;
- Agriculture: Salt is used in agricultural practices, such as in saline agriculture where salt-tolerant crops are cultivated in areas with saline soil or water [3];
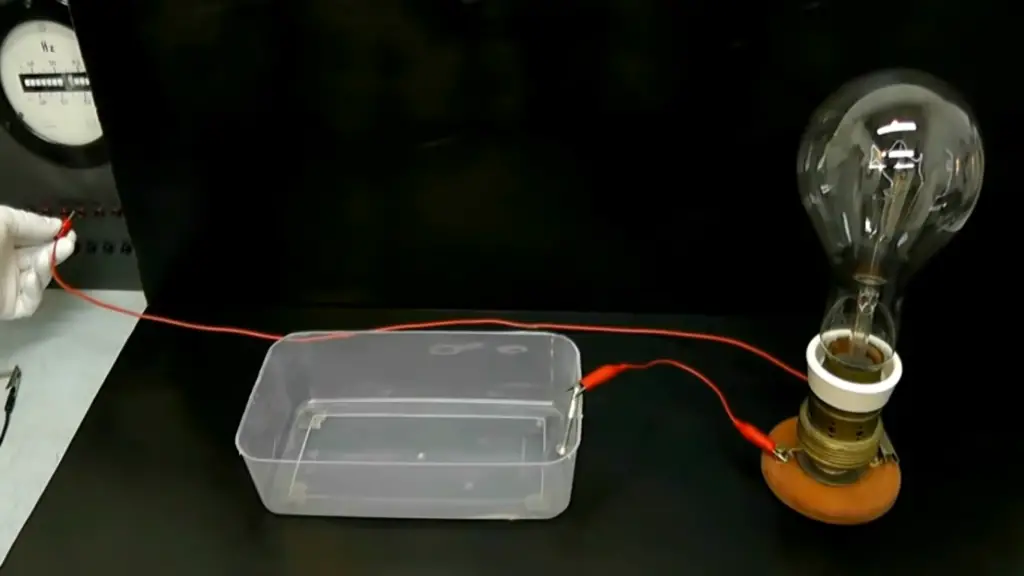
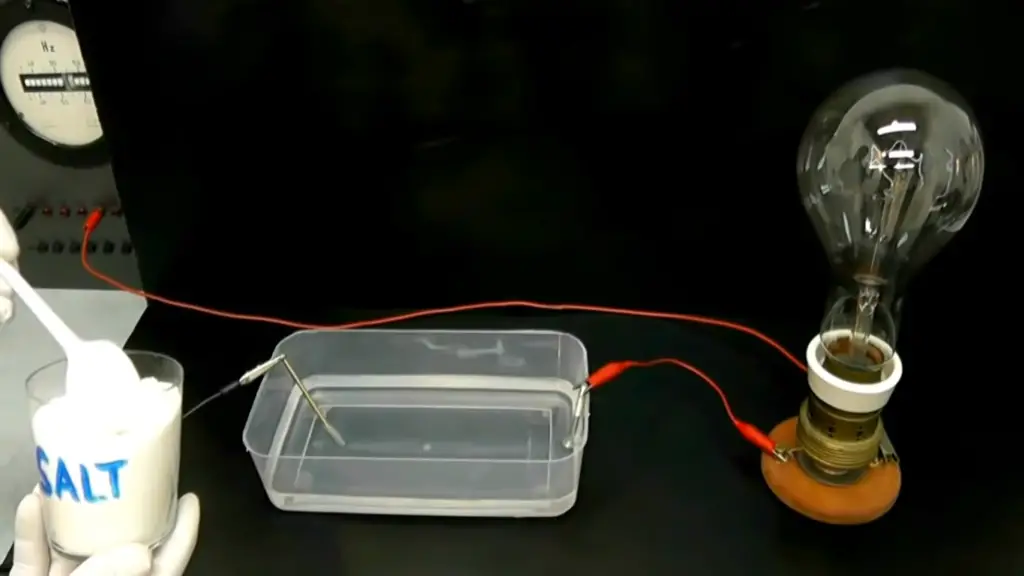
Is Salt A Conductor Or An Insulator?
Conductivity of Salt
Salt is an ionic compound, meaning it consists of positively charged ions (sodium ions – Na+) and negatively charged ions (chloride ions – Cl-). When salt dissolves in water or any other polar solvent, it undergoes a process called dissociation. During dissociation, the ionic bonds between sodium and chloride ions break, and the ions separate, becoming mobile within the solution.
Insulating Properties of Salt
In its solid crystalline form, salt is an insulator. The ionic lattice structure that salt adopts in its solid state prevents the movement of charged ions. The tightly packed arrangement of ions makes it difficult for them to move freely, restricting the flow of electricity. As a result, solid salt does not conduct electricity and acts as an insulator.
Practical Applications:
The dual nature of salt’s conductivity finds practical applications in various fields:
- Electrolytes and Batteries: Salt is used as an electrolyte in various batteries and electrochemical cells. In these applications, salt dissolved in water or another suitable solvent facilitates the movement of ions between the electrodes, allowing the flow of electrons and producing an electrical current;
- Water Treatment: Salt is used in water treatment processes, such as water softening. In this application, salt-based ion exchange resins remove calcium and magnesium ions from hard water, replacing them with sodium ions;
- De-icing Roads: During winter, salt is spread on icy roads to help melt the ice. When salt dissolves in the thin layer of water created by the melting ice, it lowers the freezing point of the water, preventing further ice formation;
- Food Preservation: Salt has been used for centuries as a food preservative due to its ability to draw out moisture from food, creating an inhospitable environment for bacteria and microorganisms;
Salt And Electricity Conductivity
Why Does Salt Conduct Electricity?
To understand why salt conducts electricity, we need to delve into its molecular structure and the behavior of its constituent ions. Salt is an ionic compound, composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-).

In its solid crystalline form, the ions are tightly packed in an orderly ionic lattice, which prevents them from moving freely. As a result, solid salt does not conduct electricity and acts as an insulator.
However, when salt is dissolved in water or any other polar solvent, a fascinating process known as dissociation occurs. The polar nature of the water molecules interacts with the ions, weakening the ionic bonds in the salt crystal. As a consequence, the sodium and chloride ions separate from each other, becoming mobile within the solution.
The presence of free-moving charged particles in the solution allows it to conduct electricity. When an electric potential is applied across the salt solution, the positively charged sodium ions move toward the negative electrode (cathode), while the negatively charged chloride ions move toward the positive electrode (anode). This movement of ions constitutes an electric current, and thus, the salt solution acts as a conductor of electricity.
Is Salt a Conductor of Heat?
In addition to its electrical conductivity, salt also exhibits thermal conductivity. However, the thermal conductivity of salt is relatively low compared to metals and other highly conductive materials. In its solid crystalline state, salt’s closely packed ionic lattice hinders the efficient transfer of heat. Therefore, solid salt is considered a poor conductor of heat [4].
Does Table Salt Conduct Electricity?
Table salt, which is the most common form of salt used in households and the food industry, is indeed capable of conducting electricity, but primarily when it is dissolved in water. In its solid form, table salt does not conduct electricity due to the reasons mentioned earlier—its ionic lattice structure restricts the movement of ions, preventing the flow of electric current.
However, when table salt dissolves in water, the dissociation of sodium and chloride ions occurs, and the resulting salt solution gains the ability to conduct electricity. This property of table salt is essential in various culinary applications, as it allows for the enhancement of flavor and food preservation.
Does Molten Salt Conduct Electricity?
Molten salt refers to a salt that has been heated to its melting point, turning it into a liquid state. At elevated temperatures, the ionic lattice structure of salt breaks down, and the ions gain enough thermal energy to move more freely within the liquid. As a result, molten salt is capable of conducting electricity, similar to a salt solution.

Molten salt’s electrical conductivity is utilized in various industrial processes, especially in high-temperature applications, such as in the production of metals, as a heat transfer fluid in certain solar power systems, and in certain types of batteries.
Does Solid Salt Conduct Electricity?
As mentioned earlier, solid salt does not conduct electricity. The closely packed ionic lattice structure in its solid state restricts the movement of ions, rendering it an insulator to electrical current.
Does Salt Conduct Electricity When Dissolved in Water?
Yes, salt exhibits electrical conductivity when dissolved in water. The dissociation of the ionic compound into mobile ions allows the resulting salt solution to conduct electricity. This property is widely utilized in various applications, including the electrolysis of water, electroplating processes, and certain medical and scientific procedures.
About Salt Water Conductivity
Understanding Conductivity
Electrical conductivity refers to the ability of a material to conduct electricity. It depends on the presence of charged particles, known as ions, which are responsible for carrying electric charge within the substance. In general, materials that contain a higher concentration of ions tend to be better conductors of electricity.
In the context of saltwater, the primary ions responsible for its conductivity are sodium (Na+), chloride (Cl-), magnesium (Mg2+), and sulfate (SO42-). These ions are dissolved in water and disassociate, creating a conductive medium. The process of dissociation, wherein salt molecules separate into ions, occurs due to the polar nature of water molecules.
Why Does Salt Water Conduct Electricity?
The conductivity of saltwater is attributed to the presence of dissolved ions in the solution. Saltwater is formed when salt (sodium chloride – NaCl) is dissolved in water (H2O). During this process, the salt dissociates into its constituent ions, namely positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-).
As the ions become mobile within the solution, they are free to move in response to an electric potential. When an electric current is passed through the salt water, the positive ions (cations) are attracted to the negative electrode (cathode), while the negative ions (anions) are attracted to the positive electrode (anode). This movement of ions constitutes the flow of electric charge, making saltwater conductive.

How Does Salt Water Conduct Electricity?
The mechanism behind saltwater conductivity involves the movement of ions in response to an electric field. When an external voltage is applied across the salt water, the electric field exerts forces on the charged ions, causing them to migrate towards the oppositely charged electrodes.
At the cathode (negative electrode), the positively charged sodium ions gain electrons and are reduced to form sodium metal. Meanwhile, at the anode (positive electrode), the negatively charged chloride ions lose electrons and are oxidized to form chlorine gas. This process is known as electrolysis, and it is essential in various industrial applications, including the production of chlorine gas and sodium hydroxide.
Why Does Salt Water Conduct Electricity But Salt Does Not?
The distinction between the conductive properties of salt and saltwater lies in their respective molecular structures. Solid salt, in its crystalline form, is an ionic compound where sodium and chloride ions are tightly packed in a repeating lattice structure. The rigid arrangement of ions in the solid state restricts their movement, making solid salt a poor conductor of electricity.
On the other hand, when salt is dissolved in water, the ionic bonds break, and the ions separate, resulting in a salt solution. The mobile ions in the solution can move freely in response to an electric field, leading to electrical conductivity [5].
In summary, while solid salt does not conduct electricity due to its rigid ionic lattice structure, saltwater conducts electricity because of the presence of mobile ions in the solution.
What Happens When Electricity Is Passed Through Salt Water?
When electricity is passed through saltwater, the process of electrolysis takes place, as explained earlier. Electrolysis is the decomposition of a substance through the passage of an electric current. In the case of saltwater, it involves the dissociation of sodium chloride into its constituent ions and the subsequent migration of these ions toward the respective electrodes.
At the cathode, reduction occurs, resulting in the formation of sodium metal and the release of hydrogen gas. At the anode, oxidation occurs, leading to the formation of chlorine gas. Additionally, some hydrogen ions (H+) are attracted to the anode, where they are oxidized to form oxygen gas.
The Polar Nature of Water
Water (H2O) is a unique compound due to its polar covalent bonds. The oxygen atom attracts electrons more strongly than the hydrogen atoms, leading to an unequal sharing of electrons within the molecule. As a result, the oxygen end of the water molecule has a partial negative charge (δ-) while the hydrogen end has a partial positive charge (δ+). This asymmetry creates a dipole moment within the molecule.
When salt (NaCl) is added to water, it dissociates into Na+ and Cl- ions. The partially charged ends of the water molecules surround and interact with these ions, breaking the ionic bonds and separating them from each other. This process allows ions to move freely in the solution, making saltwater conductive.

Measuring Conductivity
Conductivity is typically measured in units of Siemens per meter (S/m) or its inverse, Ohms per meter (Ω/m). The higher the conductivity value, the better the material can conduct electricity. For instance, metals such as copper and aluminum have high conductivities, making them excellent conductors for electrical currents.
The conductivity of saltwater depends on several factors, including the concentration of dissolved ions, temperature, and pressure. As the concentration of salt (salinity) increases, the number of ions also increases, leading to higher conductivity.
Applications of Saltwater Conductivity
Marine Life and Ecosystems: The conductivity of saltwater has a profound impact on marine life and ecosystems. Some marine species, such as sharks and rays, possess specialized organs called “ampullae of Lorenzini”, which can sense minute changes in electrical fields produced by other organisms. This ability helps them navigate, detect prey, and communicate with other members of their species [6]:
- Desalination: Desalination is the process of removing salt and other impurities from seawater to make it potable or suitable for various industrial applications. Understanding the conductivity of saltwater is crucial in designing efficient desalination methods, such as reverse osmosis and distillation;
- Oceanography and Climate Studies: Measuring the conductivity of seawater is a vital part of oceanographic research. By monitoring conductivity levels, scientists can gather data on ocean circulation patterns, salinity variations, and other essential factors that influence climate and weather patterns;
- Electrolysis: Electrolysis is a process that uses an electric current to drive a chemical reaction. Saltwater’s conductivity allows it to be used as an electrolyte in various applications, such as metal plating, electrorefining, and the production of chlorine gas and sodium hydroxide;
- Submarine Communication: Seawater’s conductivity enables the transmission of electrical signals through water. This property is crucial in submarine communication systems, where electrical signals travel through the conductive seawater to facilitate communication between submarines and onshore stations;

Challenges of Saltwater Conductivity
While saltwater’s electrical properties offer numerous benefits, they also present some challenges in certain scenarios:
- Corrosion: The high conductivity of salt water can accelerate the corrosion of metal structures, particularly in marine environments. This corrosion poses a significant concern for offshore installations, ships, and coastal infrastructure;
- Electrical Safety: Swimming in the sea during thunderstorms or in the vicinity of electrical equipment can be hazardous due to the conductive nature of saltwater. In such situations, it is essential to be aware of potential electrical hazards;
Is Salt Water More Conductive Than Freshwater?
Yes, saltwater is more conductive than freshwater due to the presence of dissolved ions. While both saltwater and freshwater can conduct electricity, the conductivity of saltwater is significantly higher.
Freshwater contains only a small number of naturally occurring dissolved ions, whereas saltwater has a much higher concentration of ions, primarily sodium and chloride ions from the dissolved salt.
The higher concentration of ions in saltwater facilitates the flow of electric charge, resulting in enhanced electrical conductivity compared to freshwater.
Does Ionized Water Conduct Electricity?
Ionized water, also known as electrolyzed water or ionized alkaline water, is water that has been treated to increase the concentration of ions, typically by passing an electric current through it. This process involves the splitting of water molecules (H2O) into hydrogen ions (H+) and hydroxide ions (OH-).
Ionized water, particularly ionized alkaline water, is considered a conductor of electricity due to the presence of these ions. The abundance of ions in the water enhances its electrical conductivity, allowing it to conduct electricity.
Is Salt Water More Conductive Than Pure Water?
Yes, saltwater is more conductive than pure water. As discussed earlier, pure water contains a minimal concentration of naturally occurring ions, primarily resulting from the self-ionization of water molecules, where water molecules dissociate into hydrogen ions (H+) and hydroxide ions (OH-). However, this self-ionization is relatively limited, and the conductivity of pure water is relatively low.
In contrast, saltwater has a much higher concentration of ions due to the dissolved sodium chloride. The abundance of mobile sodium and chloride ions significantly increases the conductivity of saltwater compared to pure water.
Is Salt Water More Conductive Than a Sugar Solution?
Yes, saltwater is more conductive than a sugar solution. While both solutions can conduct electricity to some extent, their conductive properties differ significantly due to the nature of their solutes.
In saltwater, the dissolved sodium chloride dissociates into mobile ions, which facilitate the flow of electric charge, resulting in higher electrical conductivity [7].
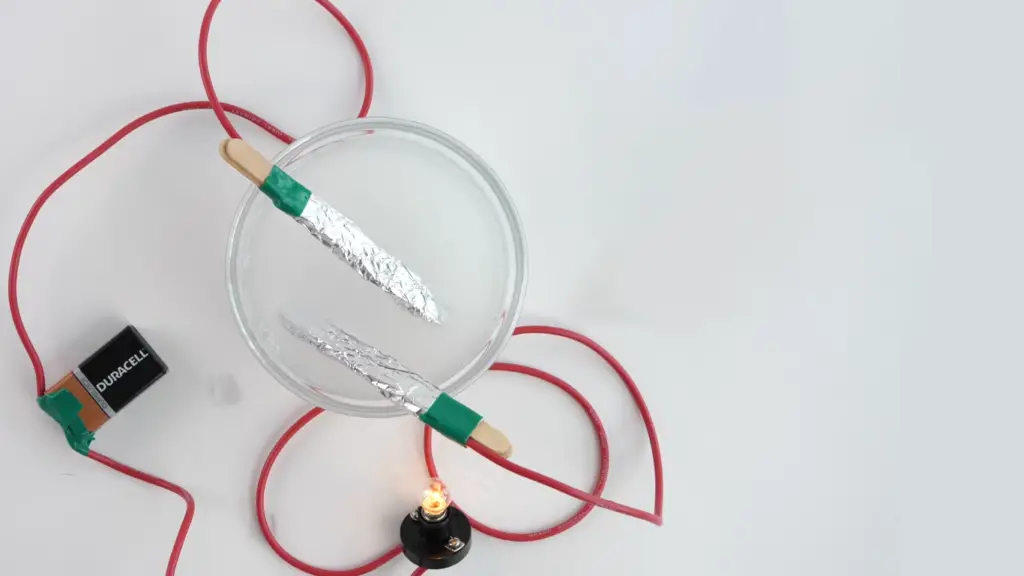
In contrast, a sugar solution, such as a solution of sucrose, does not dissociate into ions. Instead, it remains primarily as individual sugar molecules. While sugar molecules can carry a small amount of electric charge, their mobility is limited, leading to lower conductivity compared to saltwater.
FAQ:
1. Does salt water hold an electric charge?
Salt water does not hold an electric charge on its own. It is a conductive medium, which means it allows electric charge to flow through it when an electric potential is applied. When a voltage is applied across a saltwater solution, the dissolved ions (sodium and chloride ions) in the water move in response to the electric field, allowing the flow of electric charge through the solution. However, once the voltage is removed, the salt water does not retain any residual charge.
2. Why does salt water conduct electricity better than fresh water?
Salt water conducts electricity better than freshwater due to the presence of dissolved ions. Fresh water has a relatively low concentration of naturally occurring ions, mainly resulting from the self-ionization of water molecules (H2O).
On the other hand, saltwater contains a higher concentration of ions, primarily from the dissociation of salt (sodium chloride – NaCl) into sodium (Na+) and chloride (Cl-) ions. The abundance of mobile ions in salt water facilitates the flow of electric charge, leading to better electrical conductivity compared to fresh water.
3. How does salt water conduct electricity?
Salt water conducts electricity through a process called ionic conduction. When salt (NaCl) is dissolved in water, it dissociates into positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). These ions become mobile in the solution and can move in response to an electric field. When an electric potential is applied across the salt water, the ions migrate towards the oppositely charged electrodes, creating an electric current and allowing the flow of electric charge through the solution.
4. Is salt water a bad conductor?
No, salt water is not a bad conductor; in fact, it is a good conductor of electricity. The presence of dissolved ions in salt water allows it to conduct electricity efficiently, making it a conductive medium.
5. Does salt conduct heat?
Yes, salt is capable of conducting heat. However, its thermal conductivity is relatively low compared to metals and other highly conductive materials. In its solid crystalline form, salt’s ionic lattice structure restricts the efficient transfer of heat, making it a poor conductor of heat.
6. What is the conductivity of salt water?
The conductivity of salt water varies depending on factors such as the concentration of dissolved salt and the temperature of the water. Generally, the electrical conductivity of salt water is in the range of approximately 4 to 6 Siemens per meter (S/m) at room temperature.
7. Is saltwater more conductive than water?
Yes, saltwater is more conductive than pure water. As mentioned earlier, saltwater’s higher conductivity is due to the presence of dissolved ions, primarily sodium and chloride ions, which facilitate the flow of electric charge.
8. Can salt water power a light bulb?
Saltwater alone can’t directly power a light bulb as it requires an electric current to do so. However, by constructing a simple saltwater battery or saltwater circuit using electrodes and an external voltage source, it is possible to generate enough electric current to light up a small light bulb or power other low-power devices.
9. Is saltwater magnetic?
No, salt water is not magnetic. It does not possess magnetic properties on its own. However, it can conduct electricity and interact with magnetic fields when an electric current flows through it.
10. How much salt does it take to make water conductive?
The amount of salt required to make water conductive depends on the desired level of conductivity. Generally, a small amount of salt, such as a few grams per liter of water, is enough to achieve significant electrical conductivity.
11. Which salt conducts electricity best?
Among common salts, sodium chloride (NaCl), which is table salt, conducts electricity well. Potassium chloride (KCl) is another salt that exhibits good electrical conductivity.
12. Is iodized salt more conductive than other salt types?
The presence of iodine in iodized salt does not significantly impact its electrical conductivity. The primary conducting factor in salt is the dissociation of sodium chloride into sodium and chloride ions. Therefore, the electrical conductivity of iodized salt is similar to that of regular table salt.
13. How to build a saltwater circuit?
To build a simple saltwater circuit, you will need a container of salt water, two electrodes (e.g., metal strips or wires), and an external voltage source (e.g., a battery). Place the electrodes in the saltwater solution, making sure they are not touching each other. Connect one electrode to the positive terminal and the other to the negative terminal of the voltage source. The saltwater will allow the flow of electric charge between the electrodes, completing the circuit.
14. Can seawater produce electricity?
Yes, seawater can be used to produce electricity through various methods such as wave energy, tidal energy, and salinity gradient power. These renewable energy technologies harness the natural movements and salinity differences in seawater to generate electric power.
15. Can NaCl conduct electricity?
Yes, NaCl (sodium chloride) can conduct electricity. When dissolved in water, NaCl dissociates into sodium and chloride ions, allowing it to conduct electricity through ionic conduction.
16. Can you make a battery out of salt water?
Yes, you can create a simple saltwater battery by using a saltwater solution as the electrolyte between two electrodes. The chemical reactions at the electrodes will generate an electric current, allowing the battery to power low-power devices.
17. Is salt water a better conductor than copper?
No, salt water is not a better conductor than copper. Copper is an excellent conductor of electricity, and it is one of the most commonly used materials for electrical wiring and conductors due to its high electrical conductivity. Saltwater, while conductive, has much lower electrical conductivity than copper.
18. Can salt water and a magnet produce electricity?
Saltwater and a magnet alone cannot produce electricity. However, if a saltwater solution is used as an electrolyte in a battery and a magnet is involved in the setup (e.g., in a generator or dynamo), it can induce electric currents in nearby conductive materials.
19. Is rainwater a good conductor of electricity?
Rainwater is a relatively poor conductor of electricity in its pure form. However, as rainwater falls through the atmosphere and interacts with airborne particles and pollutants, it can pick up dissolved ions, making it slightly more conductive.
20. Which has the highest conductivity of water?
Among common types of water, seawater has the highest electrical conductivity due to its high concentration of dissolved salts and ions.
21. Are salt batteries real?
Yes, saltwater batteries are real and are being developed as a form of renewable energy storage. These batteries use salt water as the electrolyte to store and release electrical energy.
22. Why is salt water bad for batteries?
While saltwater can be used as an electrolyte in certain batteries, it is generally not suitable for standard commercial batteries. The corrosive nature of saltwater can damage the internal components of conventional batteries, leading to decreased performance and reduced lifespan.
Useful Video: Electrical conductivity with salt water
References
- https://www.circuitsgallery.com/is-salt-water-conductive/
- https://lambdageeks.com/does-salt-water-conduct-electricity/
- https://sciencing.com/make-negatively-charged-water-12008287.html
- https://eartheclipse.com/science/misc/does-salt-conduct-electricity.html
- https://biotrux.com/is-salt-conductive/
- https://www.homesciencetools.com/article/saltwater-circuit-project
- https://physics.stackexchange.com/questions/90932/can-a-salt-water-solution-conduct-electricity-forever














Leave a Reply